Tauramox™
(moxidectin) Injectable Solution
Tauramox™ (moxidectin) Injectable Solution is the first generic Cydectin® (moxidectin) Injectable for the treatment of infections and infestations due to internal and external parasites in beef and non-lactating dairy cattle. Producers can expect the same weight gain advantage as with Cydectin® Injectable, but at a lower cost.
Target Species: Beef cattle, Non-Lactating dairy cattle
Product Attributes
-
Ready-to-use injectable parasiticide containing 1% moxidectin
-
Treats and controls gastrointestinal roundworms, lungworms, cattle grubs, mites and lice
-
Proven to effectively protect cattle from reinfection of key internal parasites*
-
21-day pre-slaughter withdrawal
-
Injectable formulation offers flexibility when treating external parasites
-
Dung beetle friendly1
-
FDA approved
-
Available in 500 mL amber glass bottle
* Moxidectin Injectable has been proven to effectively protect cattle from reinfection with Dictyocaulus viviparus and Oesophagostomum radiatum for 42 days, Haemonchus placei for 35 days, and Ostertagia ostertagi and Trichostrongylus axei for 14 days.
1Jacobs, C.T. & Scholtz, C.H., 2015, ‘A review on the effect of macrocyclic lactones on dung-dwelling insects: Toxicity of macrocyclic lactones to dung beetles’, Onderstepoort Journal of Veterinary Research 82(1), Art. #858, 8 pages. http://dx.doi.org/10.4102/ojvr.v82i1.858
IMPORTANT SAFETY INFORMATION: Cattle must not be slaughtered for human consumption within 21 days of treatment. This drug is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. A withdrawal period has not been established for preruminating calves. Do not use in calves to be processed for veal.
Restricted Drug (California) – Use Only As Directed
-
Active Ingredient(s)
10 mg moxidectin/mL
-
Dosage Form
Ready-to-use sterile injection
-
Indications
Tauramox™ Injectable, when administered at the recommended dose level of 0.2 mg/2.2 lb (0.2 mg/kg) body weight, is effective in the treatment and control of the following internal and external parasites of cattle:
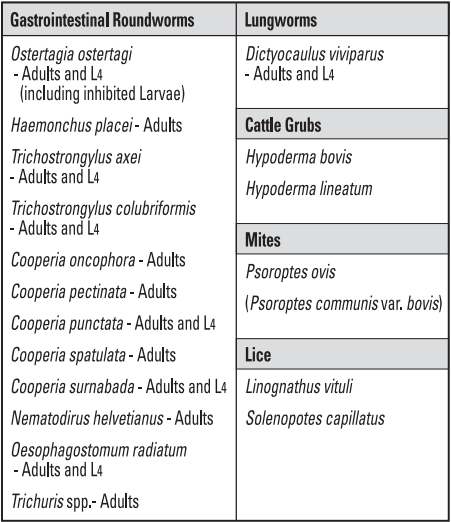
-
Dosage and Administration
DOSAGE
The recommended rate of administration for Tauramox™ Injectable is 1 mL for each 110 lb (50 kg) body weight to provide 0.2 mg moxidectin/2.2 lb (0.2 mg/kg) body weight. The table below will assist in the calculation of the appropriate volume of injectable which must be administered based on the weight of animal being treated. Be careful not to overdose animals; estimate animal’s body weight as closely as possible or weigh animals individually.
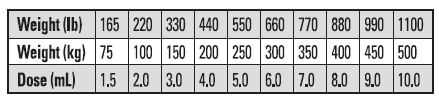
* Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment and encourage the development of parasite resistance.
ADMINISTRATION
Tauramox ™ Injectable should be administered by subcutaneous injection under the loose skin in front of or behind the shoulder (Figure 1). Needles ½ to ¾ inch in length and 16 to 18 gauge are recommended for subcutaneous injections. Use sterile, dry equipment and aseptic procedures with withdrawing and administering Tauramox™.

-
Storage
Store at 59° to 77°F (15° to 25°C). Exposure to temperature up to 104°F (40°C) may be tolerated provided the mean kinetic temperature does not exceed 77°F (25°C). Use within 3 months of first puncture and puncture a maximum of 56 times. If more than 56 punctures are anticipated, the use of multi-dosing equipment is recommended. If using a needle or draw-off spike larger than 16 gauge, discard any remaining product immediately after use.
© 2023 Norbrook Laboratories Limited. Tauramox and the Norbrook logo are registered trademarks of Norbrook Laboratories Limited. Cydectin is a trademark of Elanco or its affiliates.

Prefer to speak directly to a member of our team?
Norbrook®, Inc.
We are open Mon - Fri 8:00am - 5:00pm CST
Please note: Product information presented on this website is intended only as a brief summary of Norbrook products for your convenience. Not all products or indications are licensed in every country and may be subject to further local variations. For specific product information you should always consult a healthcare professional from your region or visit the local government agency website for the most up to date information. Please see our terms and conditions for further information.










