Durvet
Firox®
(firocoxib) Chewable Tablets
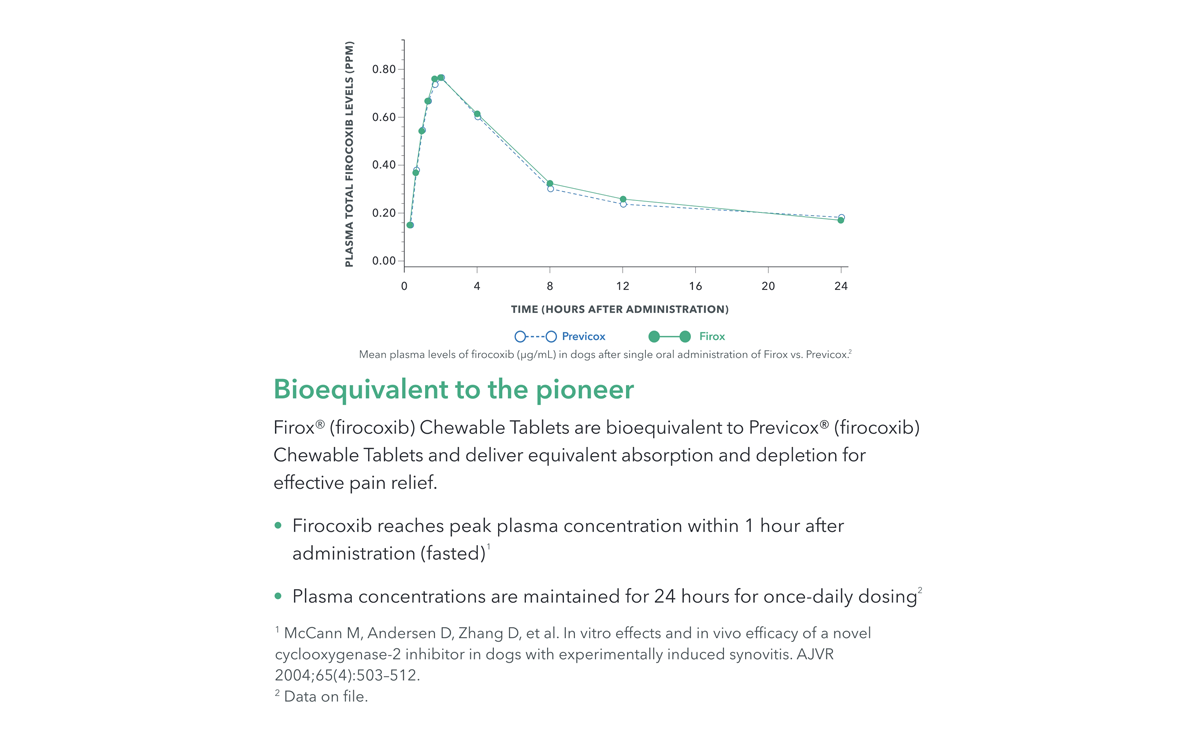
Firox® (firocoxib) Chewable tablets are indicated for the control of pain and inflammation associated with osteoarthritis and for the control of postoperative pain and inflammation associated with soft-tissue and orthopedic surgery in dogs. Firox® Chewable Tablets are bioequivalent to Previcox® (firocoxib) Chewable Tablets and deliver the same absorption and depletion for effective pain relief.
-
Firocoxib reaches peak plasma concentration within 1 hour after administration (fasted)1
-
Plasma concentrations are maintained for 24 hours for once-daily dosing2
When compared to the pioneer, Firox® is a cost-effective alternative that makes it easier to boost your profit potential while also passing greater savings along to your clients.
Target Species: Dogs
Product Attributes
-
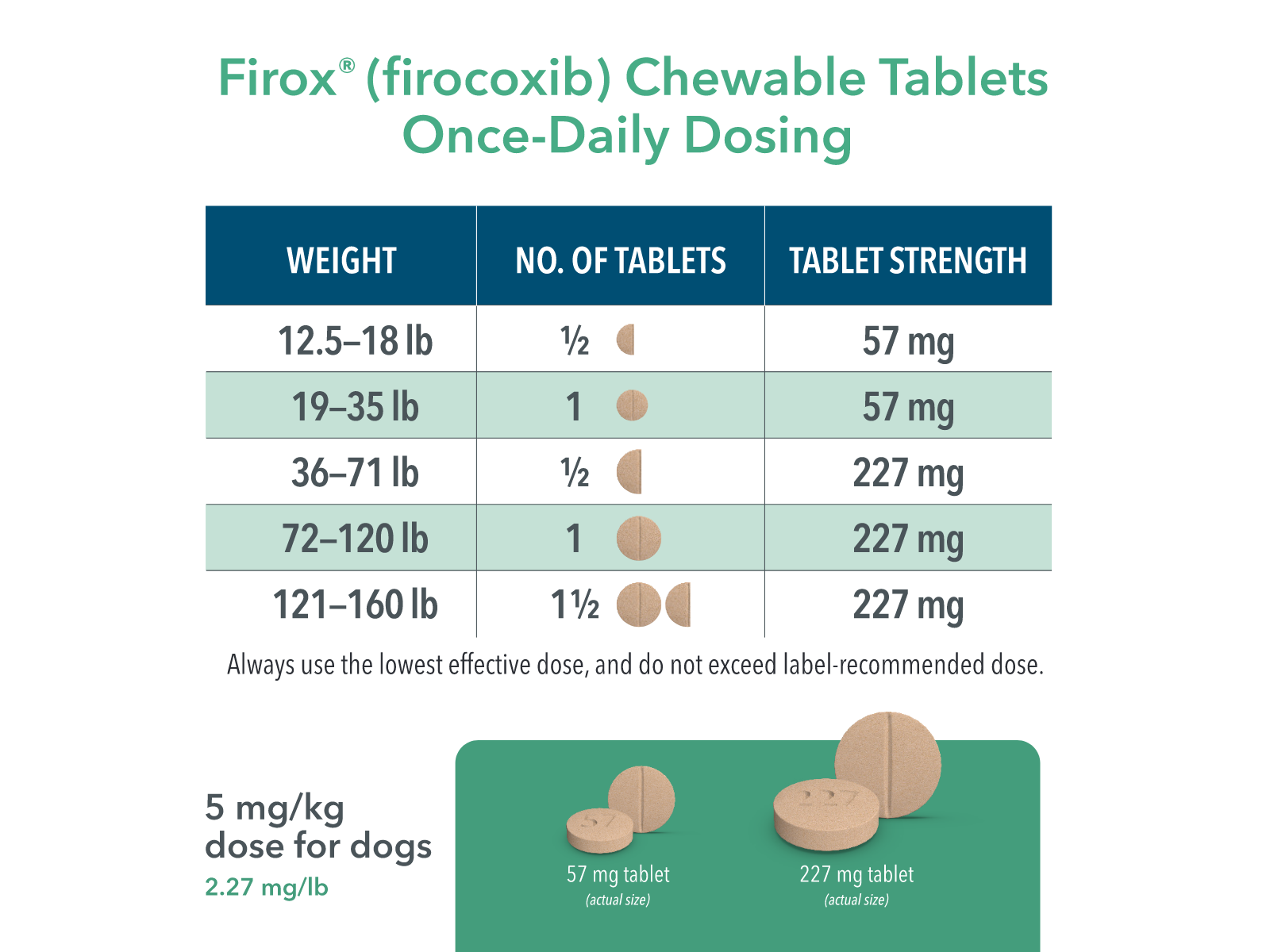
Scored for easy splitting and accurate dosing of 5 mg/kg (2.27 mg/lb) body weight, once daily
-
Chewable tablet with pork-liver flavoring
-
Can be given with or without food
-
Less expensive than Previcox® Chewable Tablets, offering significant savings and improved clinic profit potential
-
Available in 57 mg and 227 mg strengths
-
Available in 60 count and economical 180 count bottles
Proven Pain Relief
In a study of 1,000 dogs diagnosed with osteoarthritis (OA), 93% of dog owners rated their dog as “improved” and 86% of dog owners rated their dog as “happier” or “more active” after treatment with firocoxib.3
1McCann M, Andersen D, Zhang D, et al. In vitro effects and in vivo efficacy of a novel cyclooxygenase-2 inhibitor in dogs with experimentally induced synovitis. AJVR 2004;65(4):503–512.
2 Data on file.
3Ryan WG, Moldave K, Carithers D. Clinical effectiveness and safety of a new NSAID, firocoxib: a 1,000 dog study. Vet Ther. 2006;7(2):119–126.
IMPORTANT SAFETY INFORMATION: FIROX (firocoxib) Chewable Tablets are for use in dogs only. As a class, cyclooxygenase inhibitory NSAIDs like FIROX may be associated with gastrointestinal, kidney, or liver side effects. Dogs should be evaluated for pre-existing conditions and currently prescribed medications prior to treatment with FIROX, then monitored regularly while on therapy. Concurrent use with another NSAID, corticosteroid, or nephrotoxic medication should be avoided or monitored closely. For more information, please see full prescribing information.
-
Active Ingredient(s)
firocoxib
-
Dosage Form and Description
Firox® is available as round, beige to tan, half-scored tablets in two strengths, containing 57 or 227 mg firocoxib.
-
Indications
Firox® (firocoxib) Chewable Tablets are indicated for the control of pain and inflammation associated with osteoarthritis and for the control of postoperative pain and inflammation associated with soft-tissue and orthopedic surgery in dogs.
-
Dosage and Administration
The recommended dosage of Firox® (firocoxib) for oral administration in dogs is 2.27 mg/lb (5.0 mg/kg) body weight once daily as needed for osteoarthritis and for 3 days as needed for postoperative pain and inflammation associated with soft-tissue and orthopedic surgery. The dogs can be treated with Firox® approximately two hours prior to surgery. The tablets are scored and dosage should be calculated in half tablet increments but should not exceed the maximum daily recommended dose. Firox® Chewable Tablets can be administered with or without food.
-
Storage
Store below 86°F (30°C). Brief excursions up to 104°F (40°C) are permitted. Use half tablet within 90 days.
© 2023 Norbrook Laboratories Limited. The Norbrook logo and Firox are registered trademarks of Norbrook Laboratories Limited. Previcox is a registered trademark of Boehringer Ingelheim Animal Health USA Inc.
If you are a pet owner looking to purchase Firox® Chewable Tablets, please consult with your local veterinarian.
Order Firox Today
Select your preferred distributor.
First Veterinary Supply
Midwest Veterinary Supply
Penn Veterinary Supply
Victor Medical Co.

Prefer to speak directly to a member of our team?
Norbrook®, Inc.
We are open Mon - Fri 8:00am - 5:00pm CST
Please note: Product information presented on this website is intended only as a brief summary of Norbrook products for your convenience. Not all products or indications are licensed in every country and may be subject to further local variations. For specific product information you should always consult a healthcare professional from your region or visit the local government agency website for the most up to date information. Please see our terms and conditions for further information.