Animal Health International
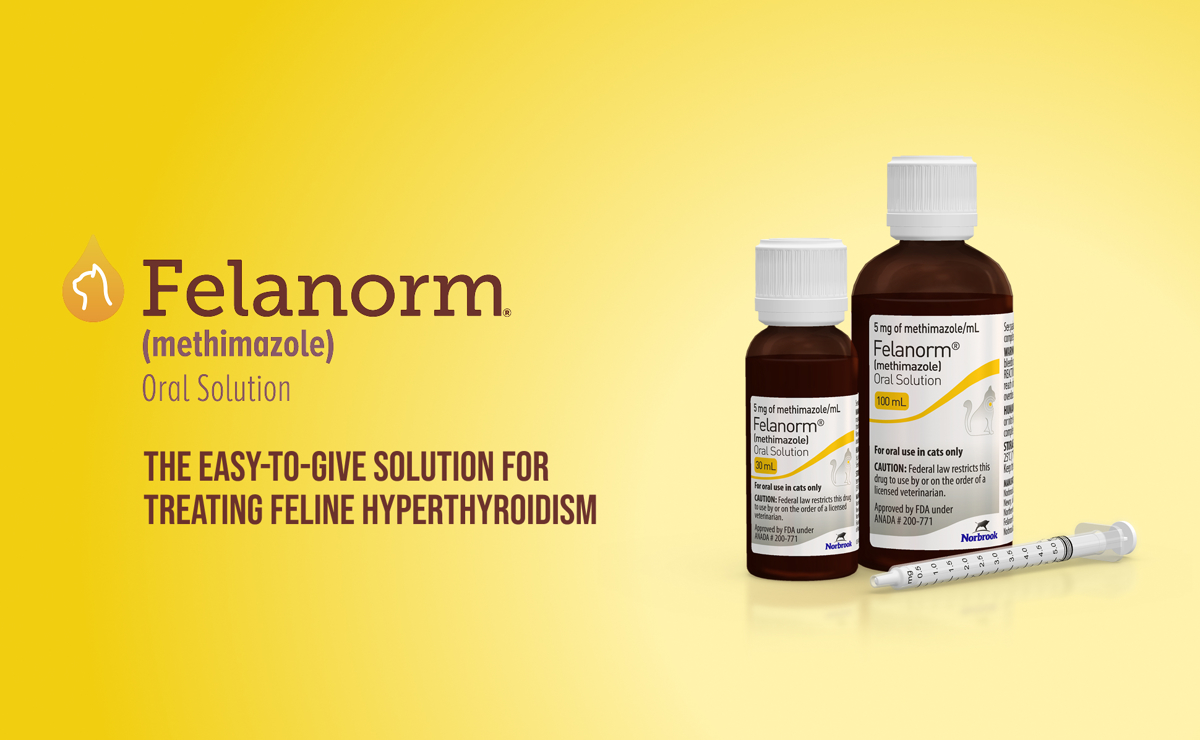
Felanorm®
(methimazole) Oral Solution 5 mg/mL
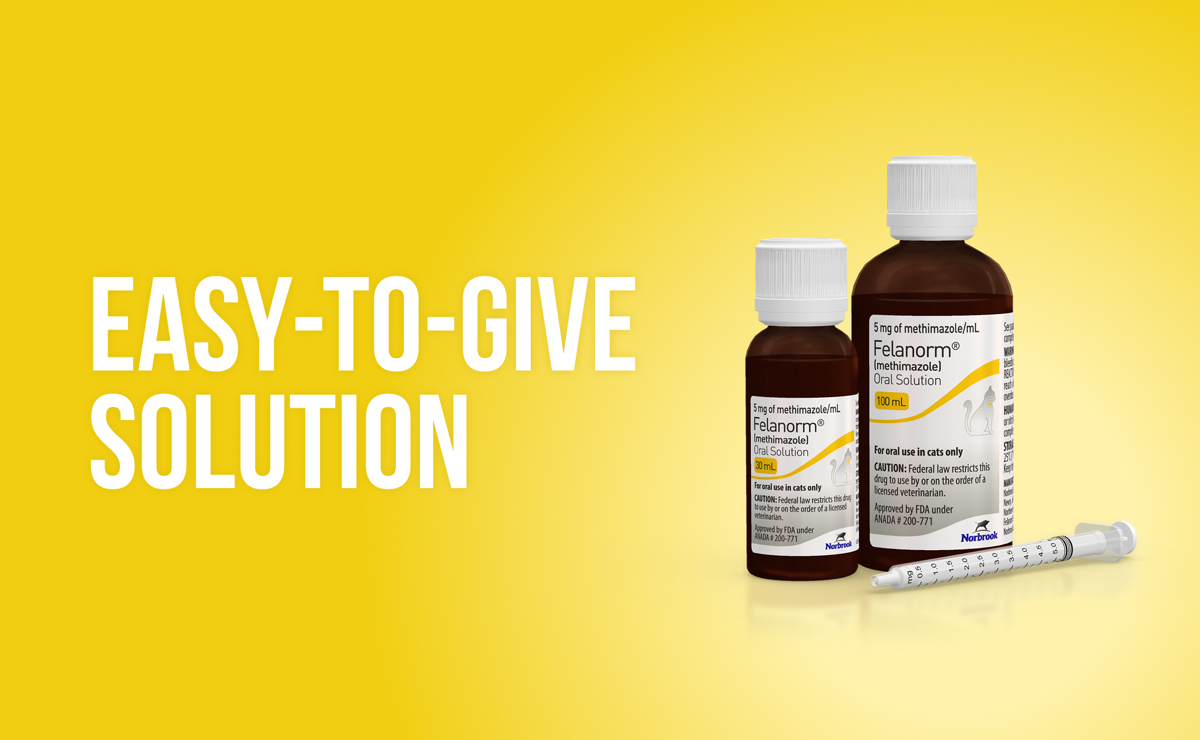
The easy-to-give solution for treating feline hyperthyroidism
No pill. No gel. No worries! Felanorm® (methimazole) Oral Solution 5 mg/mL for cats is an FDA-approved, easy-to-administer treatment for feline hyperthyroidism.
Target Species: Cats
Easy to Give. Easy to Trust.
-
The first FDA-approved oral solution for feline hyperthyroidism
-
Syringe marked in 0.25 mg increments for accurate dose titration
-
Proven track record (over 100 million sold doses in the U.K.)1
-
Easy-To-Give (International Society of Feline Medicine awarded)2
REFERENCES:
1 Kynetec Sales Data, U.K. (based on standard 2.5 mg dose).
2 International Society of Feline Medicine Easy-To-Give award 2017 (NOTE: Thyronorm® is the U.K. brand name for Felanorm; both share the exact same formulation).
IMPORTANT SAFETY INFORMATION:
CAUTION: As with all drugs, side effects may occur. The most commonly reported side effects are anorexia, vomiting, head/facial pruritus or edema, depression/lethargy, weight loss, anemia, elevated liver enzymes, skin lesions, elevated BUN, diarrhea, and thrombocytopenia. Felanorm® Oral Solution is not for use in pregnant or lactating queens, or cats with renal, hepactic, or hematological disorders. In some reported cases, the patients recovered after adverse signs were recognized, the drug was withdrawn, and veterinary care was applied. In some cases, death (or euthanasia) has been reported as an outcome of the adverse reactions listed above. Methimazole has anti-vitamin K activity and may induce bleeding diathesis without evidence of thrombocytopenia. Refer to the prescribing information for complete details.
-
Active Ingredient(s)
Methimazole is a thioureylene antithyroid drug, which inhibits the synthesis of thyroid hormones. Methimazole (1-methylimidazole-2-thiol) is a white crystalline substance that is freely soluble in water. The chemical formula is C4H6N2S. Molecular weight is 114.16.
-
Dosage Form and Description
Felanorm is available at 5 mg/mL in bottles containing 30 or 100 mL with 1 mL dosing syringe.
-
Indications
Felanorm (methimazole) is indicated for the treatment of hyperthyroidism in cats
-
Dosage and Administration
Always include the Instructions for Preparing a Dose of Felanorm (methimazole) Oral Solution and the Information for Cat Owners with a prescription. The starting dose of Felanorm is 2.5 mg administered every 12 hours. Following 3 weeks of treatment, the dose should be titrated to effect based on individual serum total T4 (TT4) levels and clinical response. Dose adjustments should be made in 2.5 mg increments. The maximum total dosage is 20 mg per day divided, not to exceed 10 mg as a single administration. Hematology, biochemistry, and TT4 should be evaluated prior to initiating treatment and monitored after 3 weeks and 6 weeks of treatment. Thereafter, bloodwork should be monitored every 3 months and the dose adjusted as necessary. Cats receiving doses greater than 10 mg per day should be monitored more frequently. The product should be administered orally using the enclosed graduated dosing syringe. The syringe has measurements representing the mg dose of the product. Rinse/clean the oral dosing syringe between uses. (See the Instructions for Preparing a Dose of Felanorm (methimazole) Oral Solution).
-
Storage
Store at controlled room temperature 25°C (77°F) with excursions between 15°-30°C (59°-86°F) permitted. Keep the container tightly closed.
Feline Hyperthyroidism, Methimazole for Cats, and "Felanorm® 101"
Get up to speed on the symptoms and treatment options of hyperthyroidism and learn more about the benefits of Felanorm.
© 2025 Norbrook Laboratories Limited. The Norbrook logos, Felanorm and Thyronorm are registered trademarks of Norbrook Laboratories Limited. 771-25-098
US Patent Numbers: 10,045,967, US 11,123,317 and US 11,738,005
Want more resources? Send us an email and it's all yours.
Request More ResourcesTech Bulletin (FAQ)
Below are a few of our most frequently asked questions. Download the document to see them all.
-
What are the most common signs of hyperthyroidism?
Weight loss
Increased appetite
Increased drinking and urination
Poor haircut (e.g., unkempt)
Behavioural changes such as hyperactivity, irritability, aggression or increased vocalization
Vomiting and/or diarrhea
-
What options are available for medical management?
The drug most commonly used to manage this disease in the U.S. is methimazole. It is currently available as a human generic tablet, a veterinary-approved tablet (Felimazole® Coated Tablets [methimazole tablets]), compounded oral and transdermal gel formulations and now the first FDA-approved oral solution, Felanorm® (methimazole) Oral Solution.
-
What is Felanorm® (methimazole) Oral Solution?
Felanorm is the first FDA-approved oral solution that is indicated for the medical management of cats with hyperthyroidism. Because it is a honey-flavored oral liquid that is easy to give, it eliminates the struggle associated with trying to “pill” cats. The oral solution also avoids some of the increased health and safety hazards that are associated with exposure to transdermal gels for cat owners and other pets in the household.
-
What are the potential side effects of methimazole?
As with all drugs, side effects may occur. The most commonly reported side effects are anorexia, vomiting, head/facial pruritus or edema, depression/lethargy, weight loss, anemia, elevated liver enzymes, skin lesions, elevated BUN, diarrhea and thrombocytopenia. Felanorm® Oral Solution is not for use in pregnant or lactating queens, or cats with renal, hepatic or hematological disorders. In some reported cases, the patients recovered after adverse signs were recognized, the drug was withdrawn and veterinary care was applied. In some cases, death (or euthanasia) has been reported as an outcome of the adverse reactions listed above. Methimazole has anti-vitamin K activity and may induce bleeding diathesis without evidence of thrombocytopenia. Please refer to the product insert for a complete list of warnings, potential side effects and prescribing information associated with Felanorm. To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Norbrook at 1-866-591-5777.
For additional information about adverse drug experience reporting for animal drugs, contact the FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
-
Are there human health risks with methimazole?
This product is not for use in humans. Keep out of reach of children. For use in cats only. Wear protective single use, impermeable (e.g., latex or nitrile) gloves when administering the solution. Wash hands with soap and water after administration to avoid exposure to drug. Wear protective gloves to prevent direct contact with litter, feces, urine or vomit of treated cats, and the solution. Wash hands after contact with the litter of treated cats.
Methimazole is a human teratogen and crosses the placenta, concentrating in the fetal thyroid gland. There is also a high rate of transfer into breast milk. Pregnant women, or women who may become pregnant, and nursing mothers should wear gloves when handling the solution, litter or bodily fluids of treated cats. Individuals with an endocrine disorder that could be impacted by methimazole should use similar precautions. Methimazole may cause vomiting, gastric distress, headache, fever, arthralgia, pruritus, and pancytopenia. In the event of accidental ingestion/overdose, seek medical advice immediately and show the product label to the physician. Avoid skin and oral exposure, including hand-to-mouth contact. Wash any spillages or splatter from the skin immediately. Do not eat, drink, smoke/vape or use smokeless tobacco while handling the product or used litter. Felanorm may cause skin or eye irritation. Avoid eye contact, including hand-to eye contact. In case of accidental eye contact, rinse eyes immediately with clean running water. If irritation develops, seek medical advice. Please refer to the product insert for a complete list of warnings, potential side effects and prescribing information associated with Felanorm.
If you are a pet owner looking to purchase Felanorm®, please consult with your local veterinarian.
News & Insights on Feline Hyperthyroidism
Order Felanorm® Today
Select your preferred distributor.
Covetrus
Durvet
First Veterinary Supply
HSB Veterinary Supply, Inc.
Midwest Veterinary Supply
Patterson Veterinary
Penn Veterinary Supply

Prefer to speak directly to a member of our team?
Norbrook®, Inc.
We are open Mon - Fri 8:00am - 5:00pm CST
Please note: Product information presented on this website is intended only as a brief summary of Norbrook products for your convenience. Not all products or indications are licensed in every country and may be subject to further local variations. For specific product information you should always consult a healthcare professional from your region or visit the local government agency website for the most up to date information. Please see our terms and conditions for further information.