Norbrook® Launches Felanorm® (methimazole) Oral Solution, Providing Veterinarians and Cat Owners an Effective and Convenient Alternative to Treating Feline Hyperthyroidism.
Lenexa, Kansas – Norbrook Inc., dedicated to providing quality and more economically priced veterinary pharmaceuticals, today announced it has released Felanorm® (methimazole) Oral Solution, the first FDA approved oral methimazole solution. Felanorm® builds on Norbrook’s strategy to launch highly differentiated products in key markets. The new oral solution is indicated for the treatment of hyperthyroidism in cats. Felanorm® has the same safety, efficacy and indications as the pioneer tablets, but is in a much more convenient formulation for ease of administration. Felanorm® (methimazole) Oral Solution will be a welcome complement to Norbrook’s current companion animal portfolio which include brands Carprieve® (carprofen) Caplets, Chewable Tablets, and Injection , Loxicom® (meloxicam) Oral Suspension for dogs, Loxicom® (meloxicam) Injection for cats and dogs, Firox® (firocoxib) Chewable Tablets, Selarid® (selamectin) for Cats and Dogs, Midamox® (imidacloprid/moxidectin) for Cats and Dogs, Enroflox® (enrofloxacin) Injection for Dogs 2.27% and Enroflox® (enrofloxacin) Chewable Tablets for cats and dogs.
“Feline hyperthyroidism is one of the most common feline endocrine diseases and occurs in ~10% of the feline population over 10 years of age. Traditional methods of medical management include oral tablets and transdermal gels. Having an FDA approved easy to give oral solution should dramatically improve the experience for both cats and their owners by avoiding both the hassle of “pilling” the cat and the increased mess and safety and health concerns associated with transdermal gels,” says Dr. Scott Krick, Technical Service Veterinarian, Norbrook. “At Norbrook, we want to help control this disease while overcoming some of the usual frustrations associated with treatment.”
Dr. Krick added that “Norbrook was the first company in the world to introduce a methimazole oral solution and Norbrook’s oral methimazole brand is the market leader in Great Britain where over 100 million doses have been sold since 20161”.
Indications
Felanorm® (methimazole) Oral Solution is indicated for the treatment of hyperthyroidism in cats.
Dosing Made Easy
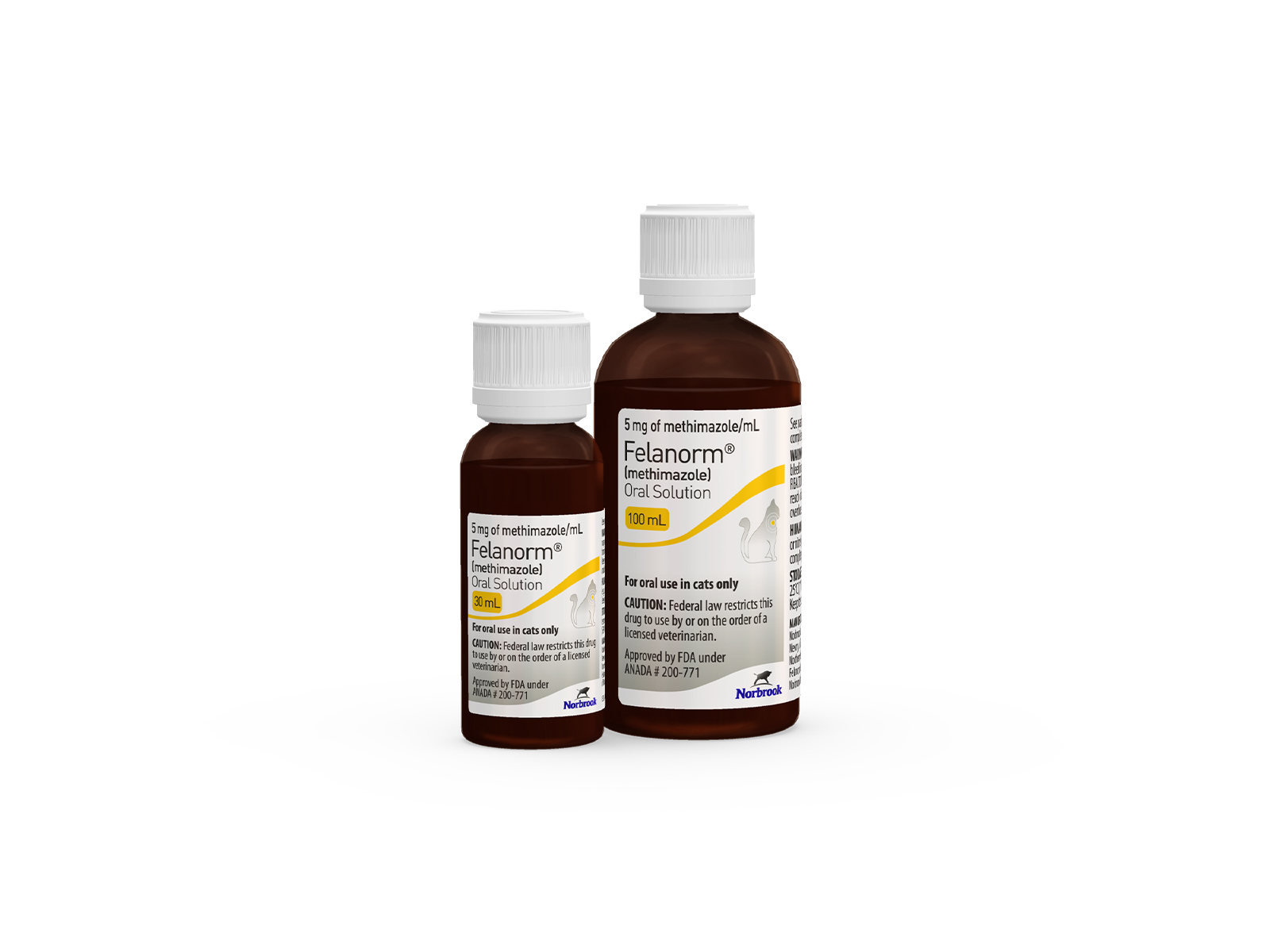
Felanorm® Oral Solution makes dosing easy for both veterinarians and cat owning clients. Available in 5 mg/mL strength, in both 30 and economical 100 mL bottles, the honey-flavored solution is readily accepted by most cats and is convenient to administer. The provided syringe is graduated in 0.25 mg increments, allowing for fine titration of dose based on response to therapy.
Affordability
While bioequivalent to Felimazole® Coated Tablets (methimazole tablets), Felanorm® Oral Solution provides a more cat-friendly route of administration. In addition, Felanorm® is more economical than compounded methimazole and provides increased revenue opportunity for veterinary clinics.
About Norbrook
Established in Northern Ireland in 1969, Norbrook is a top 20 global manufacturer of veterinary pharmaceuticals and one of the world’s largest manufacturers of veterinary sterile injectables. Norbrook products are sold in over 120 countries worldwide with global sales approaching $320M annually. A family-owned business, Norbrook continues to build for the long term and in the last number of years has invested close to $150M in capital expenditure to enhance its infrastructure and operational efficiencies.
For further information and to speak to a member of our US team, please contact: (866) 591-5777
1Kynetec Sales Data, U.K. (based on standard 2.5mg dose).
IMPORTANT SAFETY INFORMATION
CAUTION: As with all drugs, side effects may occur. The most commonly reported side effects are anorexia, vomiting, head/facial pruritus or edema, depression/lethargy, weight loss, anemia, elevated liver enzymes, skin lesions, elevated BUN, diarrhea, and thrombocytopenia. Felanorm® Oral Solution is not for use in pregnant or lactating queens, or cats with renal, hepatic, or hematological disorders. In some reported cases, the patients recovered after adverse signs were recognized, the drug was withdrawn, and veterinary care was applied. In some cases, death (or euthanasia) has been reported as an outcome of the adverse reactions listed above. Methimazole has anti‐vitamin K activity and may induce bleeding diathesis without evidence of thrombocytopenia. Refer to the prescribing information for complete details here.
US Patent Numbers: 10,045,967, US 11,123,327 and US 11,738,005
©2024 Norbrook Laboratories Limited. All rights reserved. The Norbrook logo and Felanorm are registered trademarks of Norbrook Laboratories Limited. Felimazole is a registered trademark of Dechra Pharmaceuticals Limited. 771-24-129









